Newsletter
Issue 12 Winter/Spring 2008

|
Newsletter |
 |
| CRASHING
CONTINENTS |
The theme of this newsletter is global tectonic plates – the massive chunks of the earth’s crust that grind past each other, sometimes releasing huge amounts of pent up energy. It was inspired by a minor earthquake that affected Britain last month. The earthquake measured 5.4 on the Richter Scale – small by international standards – but nevertheless the largest earthquake in Britain for 25 years. Thankfully there was only one casualty – an elderly man suffered a broken pelvis when a chimney collapsed on him. We have yet to find out whether or not he was enrolled in the CRASH-2 trial! Now that the CRASH-2 trial is truly global with collaborators from 35 countries world-wide and gradually getting up to full speed in terms of recruitment, we have divided the office work into four global regions, each with a different regional co-ordinator (Eni Balogun, Pablo Perel, Taemi Kawahara and Lisa Cook). In this newsletter each co-ordinator has written a short update about their region. I encouraged them to be as competitive as possible – pointing out how well their region is doing in terms of recruitment – in the hope that this would encourage some friendly competition and creative tension between the various tectonic plates. You can judge for yourselves, but my impression is that if our regions were tectonic plates then we would be lucky to muster a creak, let alone an quake! I realise now that the problem with my newsletter idea is that CRASH-2 is a success because of collaboration rather than competition and I suspect that regional co-ordinators understand this. Competition
leads to small clinical trials that might be good for the ego of the people involved,
but because a small group of individuals can only recruit a small number of patients
the data from these trials is unreliable, which is not in the best interests of
patients. Small trials are also vulnerable to publication bias and most doctors
never get to hear about them. The CRASH-2 trial is different – working together
we have randomised close to 9,000 patients and are well on target to reach 20,000
by the end of next year. Here is to Collaborating Continents! |
SOUTH EAST ASIA, AUSTRALIA, CHINA, GEORGIA, PAKISTAN | ||
 |  |  |
South East Asia is just now beginning to realise its potential for patient recruitment so it may well be the number one region in the world by the end of the trial. Trauma rates are high in this region, so CRASH-2 is extremely relevant. But what makes the trial a success is the commitment of the collaborators, and nowhere is this commitment stronger than in South East Asia. Indonesia currently leads the way and is one of the most successful countries in the trial even though there are only four active sites. These sites – Sanglah General Hospital, Soebandi Hospital Jember, Saiful Anwar General Hospital and Cipto Mangunkusumo Hospital – work hard to put in a phenomenal number of patients. Nyoman Golden, the Indonesia National Co-ordinator, is also currently working on expanding the number of active hospitals to keep Indonesia in the number one position. Close behind is Thailand. The trial is expanding rapidly and with the help of the National Co-ordinator Surakrant Yutthakasemsunt we have recently welcomed several new sites: Burirum, Lampang, Bhumibol Adulyadej and Pattani Hospitals. We are expecting great things of these sites and if they are to follow the strong example of the Khon Kaen and Suratthani Hospitals, Thailand could be catching up with Indonesia very soon! In Malaysia we have two University Hospitals, University of Malaya Medical Centre and Hospital University Science Malaysia, currently recruiting and hope to have a Ministry of Health approval soon so that government hospitals can also join. There is a real enthusiasm for research in Malaysia so once we have the approvals we are really expecting the trial to take off there. Georgia, for its population size, is recruiting exceedingly well. We have just welcomed two new sites: Tbilisi State University Clinical Hospital 'I Javakhishvili' and the Institute of Critical Care Medicine. These hospitals will be a great asset as our three active Georgian sites – City Hospital no.1 and Tbilisi State Medical University ER Department and Central Clinic – are always among our top recruiters. We believe recruitment will go up due to a change in law which allows randomisation without prior written consent, so expect to see these numbers to get even higher. In all these countries we have just few hospitals recruiting high numbers of patients. We need more hospitals to join who can recruit at the same rates. So Collaborators, tell your peers about CRASH-2 and keep up the excellent work – remember: EVERY PATIENT COUNTS!! Lisa Cook, Regional co-ordinator | ||
| AFRICA, MIDDLE EAST, WESTERN EUROPE | ||
 |  |
|
Hospitals
across my region account for over 35% of patients randomised into the trial. This
is an incredible achievement. WELCOME
TO THE FOLD NEARLY
THERE | ||
| UNITED
KINGDOM We now have a Nurse Coordinator Jackie Wayte in post to help with recruitment in the UK and with her support we look forward to the UK matching the recruitment of the MRC CRASH Trial – at a minimum! | CRASH-2
trial was represented at the conference of Emergency Medicine Society of South
Africa (EMSSA) in October 2007, by Tim Coats | |
|  | |
PLEASE
CONTINUE TO SPREAD THE WORD Eni Balogun, Regional co-ordinator | ||
| ||
| LATIN AMERICA AND THE CARIBBEAN REGION | ||
 |  |  |
Latin America is having a very active participation in the CRASH-2 Trial. Six countries are already taking part. More than 40 hospitals from Argentina, Colombia, Cuba, Ecuador, Mexico and Peru have already recruited 2,500 patients, almost 30% of all randomised patients. Our collaborators have presented the trial in several conferences in the region and published editorials in local journals highlighting the relevance of the CRASH-2 Trial. Our region is already one of the biggest contributors of CRASH-2 but we have the potential to further increase our participation. Unfortunately injuries are endemic in our region and this means that we have a huge potential to recruit many more patients, but also that if the trial succeeds in finding an effective treatment this will translate into an important and affordable treatment for our patients. We
encourage our collaborators to continue their participation and their advocacy
for the CRASH-2. We still Pablo Perel, Regional Co-ordinator | ||
 ECUADOR: Team at Hospital Alcivar with Daniel Acosta Farina | ||
| INDIA, SRI LANKA, NEPAL, BANGLADESH, SOUTH PACIFIC | ||
 | 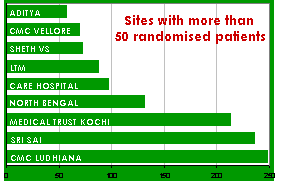 |  |
My region is doing exceptionally well having recruited 20% of the almost 9,000 patients randomised into the trial so far. We have a very high data quality with 92% complete data (i.e. fully completed EFs and OFs returned). INDIA is now the highest recruiting country in the world with the most actively recruiting Sites. This is not surprising as over 70,000 people are killed and many thousands more seriously injured each year through road traffic crashes alone. This study, if positive, will be very relevant to India’s doctors and patients. The national meeting in Cochin 16-17 February was a great success. Our top five recruiters, Yashbir Dewan, Christian Medical College Ludhiana, Sanjay Gupta, Sri Sai Hospital, R R Ravi, Medical Trust Hospital Kochi, Satish Dharap, LTM General Hospital and P V Ramana, Care Hospital, each gave an excellent presentation about how they have made the CRASH-2 trial a success. Sadly another one of our top recruiters, Wu Hoong Chhang, North Bengal Neuro Research Centre, could not make it to the meeting, but his contributions have not gone unnoticed. Since the meeting we have several collaborators who have exceeded the 50 patient mark and are on track to becoming top recruiters. M Chidambaram (Government Rajaji Hospital), Ranasanda Mangual (MKCG Medical College), Ashok Pangi (KLE Hospital & Medical Research Centre) and Satish Dharap (LTM Medical College & General Hospital) have all recently made efforts to increase their recruitment rates so thanks goes to them. As a result of the meeting six new hospitals have started randomisation and another nine are about to start. Our Indian collaborators are leading the way by showing it is possible to recruit patients in the very difficult and challenging situation soon after major injury. I hope that you will continue to recruit all eligible patients and maintain your position as top recruiters in the world! SRI
LANKA CRASH2
extends to the South Pacific Taemi Kawahara, Regional Co-ordinator | ||
 INDIA: Bhattacharya Orthopaedic and Related Research Centre with Protyush Chatterjee | ||
 INDIA: St Stephen’s Hospital with Subrat Kumar Raul | ||
 INDIA: Government Rajaji Hospital with M Chidambaram | ||
 INDIA: Aditya Neuroscience Centre with Anil P Lal | ||
| ||
milestones Many congratulations to the collaborators and their trial teams listed below for these fantastic recruitment achievements! | |
250 100 50 | |
| NEW ETHICS APPROVALS - CONGRATULATIONS! | |
INDIA
| |
| NEW RANDOMISERS | |
|
United Nations road safety resolution | |
On 31 March 2008 the United Nations General Assembly adopted a resolution aimed at alleviating the global road safety crisis. The Assembly encouraged member states to strengthen their commitments to road safety by observing the annual World Day of Remembrance for Road Traffic Victims in December, organising global road safety weeks and encouraging fleet-owning organisations in both the private and public sectors to develop and implement policies and practices that would reduce road-crash risks. The states emphasized that road traffic injuries posed a global public health crisis requiring urgent national and international action. | |
 Brigitte Chaudhry | Also introduced was a report on global road safety, prepared by WHO, in consultation with the regional commissions and other partners of the UN Road Safety Collaboration, saying it provided an update on efforts to implement Assembly recommendations, and described how collaborative efforts in the past two years had increased road safety awareness nationally and internationally. The CRASH-2 trial Steering Committee member Brigitte Chaudhry attended the Assembly and brought this report to our attention. Brigitte is the president of the European Federation of Road Traffic Victims, FEVR, and the founder and president of RoadPeace, the British charity for road crash victims. |
| CRASH2 evaluates health economics | |
I
joined the CRASH-2 team as a Health Economist in March 2008. Within this team
my primary role is to perform cost effectiveness analysis in the early administration
of antifibrinolytic in significant haemorrhage. My background is in international
economics with a MSc in Health Economics. I am very excited to start work with
the internationally based CRASH-2 team. Indeed, it is an incredible opportunity
to collaborate with experts from all over the world, to exchange ideas and make
a contribution to such an important research field. |  Carla Guerriero |
 | cochrane
corner |  |
My name is Emma Sydenham and I’m the new Review Group Co-ordinator (RGC) for the Cochrane Injuries Group (CIG). The CIG conducts and updates systematic reviews about interventions for injury prevention. As a CRASH-2 Collaborator I would like to invite you to get involved in one of the following activities: Review
Author: Author a review for The Cochrane Library! The Injuries Group
Editorial base can provide the support, resources and training to tackle a systematic
review. To see a full list of CIG reviews, please go to http://www.cochrane-injuries.Lshtm.ac.uk. You are most welcome to email me at Emma.Sydenham@Lshtm.ac.uk to find out more about the group and to get involved! | ||